Background: Despite widespread interest in pain management and opioid use across the United States, information on pain and opioid utilization in patients with relapsed or refractory acute myeloid leukemia (R/R AML) is lacking. Better understanding of patient-reported outcomes (PROs) specific to pain could be used to identify strategies to improve the quality of life in patients with R/R AML.
Aim/Objective: To describe pain and opioid use in patients with FLT3 mutation-positive (FLT3mut+)R/R AML receiving either gilteritinib or salvage chemotherapy (SC) using PRO data collected from the ADMIRAL study (NCT02421939).
Methods: ADMIRAL was a phase 3, open-label, multicenter, active-controlled randomized study comparing the efficacy and safety of gilteritinib to SC in patients with FLT3mut+ R/R AML. Pain was assessed using selected items from the Functional Assessment of Cancer Therapy - Leukemia (FACT-Leu; GP4 item: "I have pain") and the EuroQol 5-Dimension 5-Level Questionnaire (EQ-5D-5L; Pain/Discomfort domain). Data for these instruments were collected at baseline (BL), Day 1 of every treatment cycle, and end of treatment (EOT). A modified EOT (mEOT) was defined as the last PRO assessment before patient discontinuation, study data cut-off date, or patient death. Patients on high-intensity chemotherapy (HIC) were treated for up to two cycles depending on treatment response; as such, only changes from BL to Cycle 2 were evaluated. Opioid utilization, including percentage of patients using any opioid medication, specific medications, duration of use, and use by transfusion dependence, was also described. Analyses of the intention-to-treat population using analysis of covariance, including BL score, response to first-line AML therapy, and investigator-preselected SC as covariates, were conducted to estimate least squares mean (LSM) and compare the differences in pain question responses between treatment arms. Descriptive statistics were used to describe opioid utilization.
Results: Of 371 eligible patients, 247 were randomized to gilteritinib and 124 to SC. The median age for both groups was 62 years and slightly more patients were female (gilteritinib, 53.0%; SC, 56.5%). Improvements at the mEOT from BL in the Fact-Leu GP4 item were observed in both gilteritinib (LSM -0.3) and SC (LSM -0.1). Scores also changed on the EQ-5D-5L at the mEOT from BL for both groups (gilteritinib, LSM 0.2; SC, LSM 0.3). No treatment differences were observed between gilteritinib vs SC on the change from BL to Cycle 2 or mEOT on the Fact-Leu GP4 item (LSM [95% CI] of -0.1 [-0.65, 0.38]; P=0.6016 and -0.2 [-0.53, 0.21]; P=0.3902, respectively) or on the EQ-5D-5L Pain/Discomfort domain (LSM [95% CI] of 0.2 [-0.21, 0.62]; P=0.3255 and -0.1 [-0.38, 0.23]; P=0.6288, respectively).
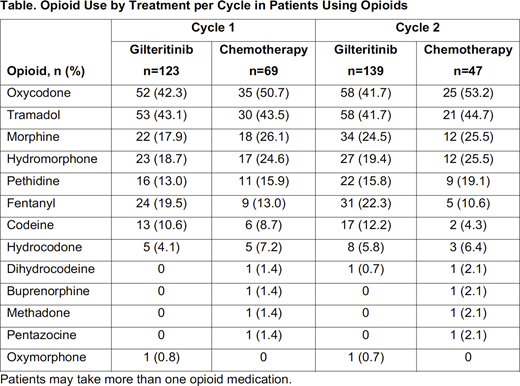
During Cycles 1 and 2, no differences were identified between gilteritinib or SC on the percentage of patients using opioids (Cycle 1: 49.8% vs 55.6%; Cycle 2: 58.9% vs 62.7%, respectively) or the time-averaged duration of use (Cycle 1: 12.4 days vs 14.1 days; Cycle 2: 15.0 days vs 17.2 days, respectively). Patients on gilteritinib were less likely to use opioids during the first two cycles compared with patients on HIC, when stratified by chemotherapy intensity (Cycle 1: 49.0% vs 72.0%, P<0.05; Cycle 2: 58.2% vs 74.1%, P<0.05). Conversely, patients on gilteritinib were more likely to use opioids compared with patients on low-intensity chemotherapy during the first two cycles (Cycle 1: 51.0% vs 30.6%, P<0.05; cycle 2: 60.0% vs 33.3%, P<0.05). In patients using opioids across the first two cycles (Table), opioids used most frequently were oxycodone (Cycle 1: 45.3%; Cycle 2: 44.6%) and tramadol (Cycle 1: 43.2%; Cycle 2: 42.5%). In patients on gilteritinib, those dependent on transfusions were generally more likely to use opioids, and for more days (time-averaged) during each cycle than patients independent of transfusions.
Conclusions: Patients with FLT3mut+ R/R AML receiving gilteritinib or SC demonstrated modest changes in responses to pain-related assessments at EOT compared with BL values. Opioids were used more frequently by patients receiving HIC regimens and transfusion-dependent patients receiving gilteritinib. These data suggest that treatments for FLT3mut+ R/R AML may impact opioid use; further study should be done to determine the relationships between these factors and their potential impact on overall quality of life.
Cella:DSI: Consultancy, Research Funding; Evidera: Consultancy; Ipsen: Consultancy, Research Funding; Mei Pharma: Consultancy; Oncoquest: Consultancy; ASAHI KASEI PHARMA CORP.: Consultancy; BMS: Consultancy, Research Funding; IDDI: Consultancy; Kiniksa: Consultancy; Novartis: Consultancy; Pfizer: Consultancy, Research Funding; Apellis: Consultancy; Alexion: Research Funding; Clovis: Research Funding; Janssen: Research Funding; Pled Pharma: Research Funding; PROMIS Health Org: Membership on an entity's Board of Directors or advisory committees, Other; BlueNote: Consultancy; Astellas: Consultancy, Honoraria; FACIT.org: Membership on an entity's Board of Directors or advisory committees, Other: President; Abbvie: Consultancy, Research Funding. Ritchie:Abbvie: Honoraria; Sierra Oncology: Honoraria; Novartis: Honoraria; Pfizer: Honoraria, Research Funding; Jazz pharmaceuticals: Honoraria, Research Funding; Incyte: Speakers Bureau. Kanda:Pfizer: Honoraria, Research Funding; Astellas Pharma: Honoraria, Research Funding; Janssen: Honoraria; Shionogi: Research Funding; Chugai Pharma: Honoraria, Research Funding; Otsuka: Honoraria, Research Funding; Celgene: Honoraria; Sumitomo Dainippon Pharma: Honoraria; Eisai: Honoraria, Research Funding; Novartis: Honoraria; Kyowa Kirin: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria; Takeda Pharmaceuticals: Honoraria; Alexion Pharmaceuticals: Honoraria; Shire: Honoraria; Daiichi Sankyo: Honoraria; Ono Pharmaceutical: Honoraria; Nippon Shinyaku: Honoraria, Research Funding; Mochida Pharmaceutical: Honoraria; Mundipharma: Honoraria; Sanofi: Honoraria, Research Funding; Meiji Seika Kaisha: Honoraria; Merck Sharp & Dohme: Honoraria. Ivanescu:Astellas: Other: IQVIA employee which is a contracted by Astellas. Pandya:Astellas Pharma, Inc.: Current Employment. Shah:Astellas: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal